Nigeria Country Profile
Nigeria
191 Million

Purpose and intended audience
Landscaping of existing Digital TB Technology tools and new relevant products that may be applied across the TB Care Cascade Model and providing insight to product developers and partners into country-specific policy, regulatory and implementation processes.
Key numbers
Country TB profile
TB Cases the Health System is Identifying:
25%
Health System Efficiency Ranking:
187 of 191
Business
Ease of Doing Business Ranking:
146 of 190
Economy
Income Category:
Upper Low Middle Income
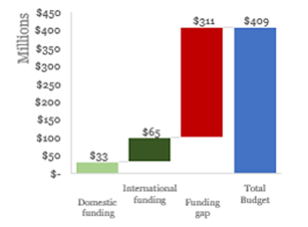
Budget & Financing
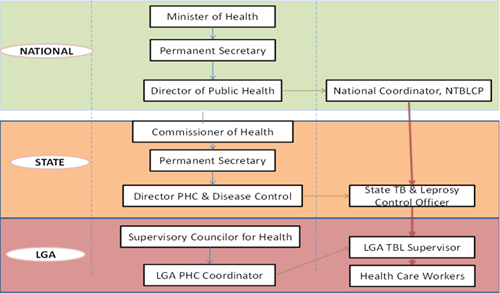
National digital TB landscape
Table 1: Digital TB Care Technology Tools currently in use
| PRODUCT NAME | DEVELOPER | USAGE |
|---|---|---|
| Diagnosis and Transport | ||
| QuanTB | MSH | Quantification & Supply Planning |
| mSupply | MSUPPLY | Inventory Managment |
| NAVISION | Microsoft | Logistics Management |
| TB-LIS | IHVN | Lab Information Management |
| Pick’NPack | NTP | Inventory Management |
| Sample Transport App | R4H | Sample Transportation |
| OneStopTB | DELFT | TB Screening |
| Early Care Seeking | ||
| GxAlert | Open Source | Connectivity |
| ASPECT | SystemOne | Connectivity |
| Triage and Adherence | ||
| NIMCURE | CcHUB | Patient adherence solutions |
| e-TB Manager | MSH | Case Management |
| MATS | Pharm Access | Linkage to care & Case Management |
| TB STARR | Abt Associate | Tuberculosis Screening and Tracking |
| ICT, Dashboard and AI | ||
| TB-SS mhealth tool | Abt Associate | Supportive Supervision |
| Open Data Kit Collect | Open Source | Data Collection |
| DHIS-2 - software | Open Source | Reporting, analysis and dissemination of health program data. |
National TB country team
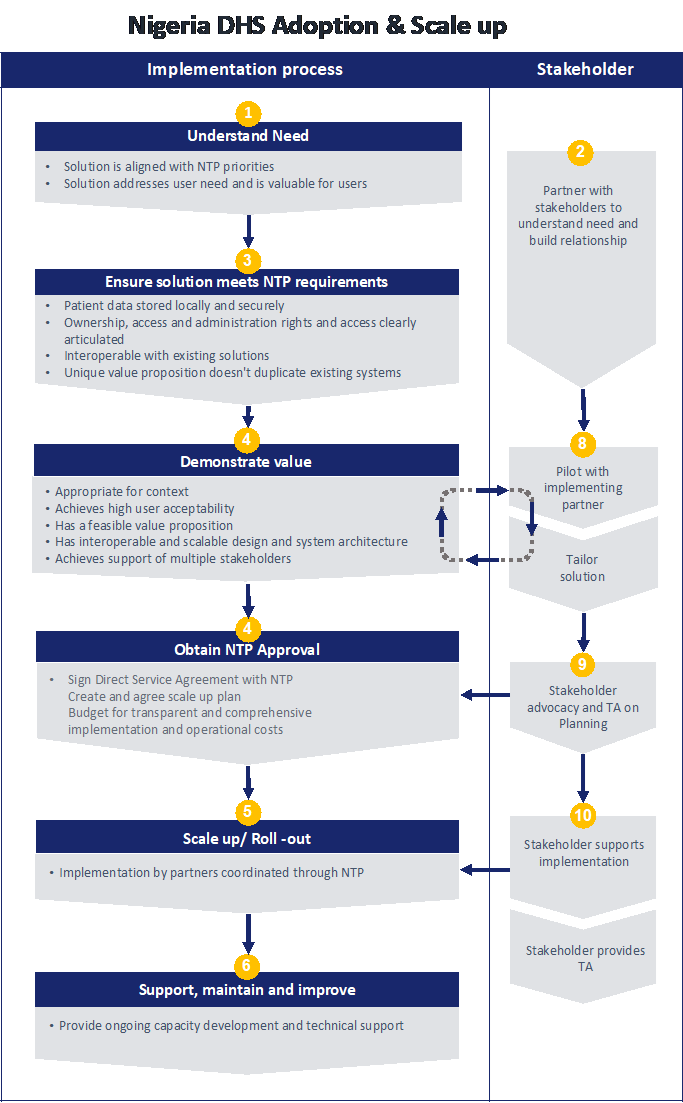
Market Entry Diagram
e-Health Policy & Regulation
The policy and regulatory environment for digital health technology in Nigeria is still at its infancy. Nigeria’s National ICT Strategic Framework (2015 – 2020), with the vision “By 2020, health ICT will help enable and deliver universal health coverage in Nigeria.” can be accessed here:
http://www.uspf.gov.ng/images/files-temp/National_Health_ICT_Strategic_Framework2015-2020-DRAFT.pdf
Registration of imported medical devices is governed by the National Agency for Food and Drug Administration and Control (NAFDAC) as described in document R&R-GDL-OO8-OO, which states that no medical device shall be manufactured, imported, exported, advertised, sold or distributed in Nigeria unless it has been registered in accordance with the provisions of Act Cap F33 LFN 2004 and the accompanying guidelines. There is, however, no specific mention of Software as a Medical Device (SaMD). In practice, the regulation process is often lax for digital solutions. None of the digital technology currently employed by the TB program has undergone the registration process.
Steps in the NAFDAC registration process include:
Application
- A written application for registration of imported medical devices should be made on the company’s letter head paper to the Director-General (NAFDAC)
- An online application form for Product Registration should be purchased at; http://registration.nafdac.gov.ng and completed.
Documentation
The following documents (all originals) and two (2) sets of photocopies (including print-out of the completed online Registration form) are to be submitted to NAFDAC
- Notarized Declaration.
- Power of Attorney or Contract Manufacturing Agreement.
- Certificate of Manufacture and Free Sale.
- Comprehensive Certificate of Analysis.
- Evidence of Business Incorporation of the importing company with the Corporate Affairs Commission in Nigeria.
- Evidence of Registration of Brand Name with Trademark Registry in the Ministry of Industry, Trade and Investment.
- Letter of Invitation for Good Manufacturing Practice (GMP) Inspection: A letter of invitation to inspect the factory abroad shall be written by the manufacturer.
Import Permit
Upon successful screening of documentation and review of supporting documents, an Import Permit shall be issued after which the product will be submitted for vetting.
Submission of products for laboratory analysis
After successful vetting of product labels, laboratory samples are submitted.
Product Approval meeting
Upon satisfactory documentation review, satisfactory GMP of the production facility and satisfactory laboratory analysis of product, products are presented for Approval Meetings.
Issuance of Notification
For products approved at the meeting, Notification of Registration or Listing is issued to the applicant while compliance directive is issued to those not approved.
Labelling for Imported Medical Devices: should be informative, accurate and in conformance with the Agency’s Medical Devices Labelling Regulations and any other relevant Regulations.
Tariff
Please refer to Tariff section.
The timeline for product registration from acceptance of submissions to issuance of Registration number is one hundred and twenty (120) working days. A successful application will be issued a Certificate of Registration with a validity period of five (5) years or two (2) years as applicable.
The NAFDAC registration guidelines for imported medical devices can be accessed here:
Registration guidelines for locally manufactured medical devices can be accessed here
PUBLIC PROCUREMENT PROCESS & TENDERS:
Details of Nigeria’s procurement and tender process can be accessed here:
http://eppms.aceondo.net/documents/procurement/procurements_manual.pdf
Operational issues & challenges
- For suppliers of technology and equipment in Nigeria, local agents and distributors with specialist industry knowledge and established networks are generally the best way to build market contacts, navigate regulatory and procurement processes and identify emerging opportunities. Services firms should consider establishing a relationship with a local business partner and/or establishing a direct presence in Nigeria.
- It is necessary to obtain government buy-in. The NTBLCP coordinates and ensures integration across the country’s TB e-health systems through the National Electronic TB Management Systems (NETIMS). NETIMS drives the agenda for how digital technology is leveraged by the TB program towards achieving program goals of eliminating TB in Nigeria. Suppliers and their in-country representatives must therefore take the time to build a relationship and ensure alignment and close collaboration with the NTBLCP’s agenda.
- The regulatory process for SaMD in Nigeria is rather ambiguous. While an e-health policy exists, it makes no mention of registration nor regulation. It is a well-known and documented fact that medical devices in Nigeria are regulated by NAFDAC, but again, there is no specific mention of SaMD. Many digital solutions currently being used in the country have not undergone NAFDAC registration.
- 84% of Nigerians use a mobile phone. This presents an opportunity for mHealth solutions, many of which have been successfully adopted in Nigeria. However, the country faces infrastructural challenges such as interruptions in power supply, Internet connectivity and mobile network coverage. Limited smart phone penetration and computer literacy of many end-users, particularly in rural areas, suggest that a coherent training plan must be an integral part of product introduction to promote uptake of technology.
- A grossly underfunded healthcare system means that public sector programs require donor funding, without which many program activities cannot be executed. Profit-based models of care that would involve out of pocket spending for TB patients is often discouraged by government, since TB disproportionately affects the poor. Suppliers must therefore explore donor-funding opportunities to ensure long term success of their digital product.
Case studies / Proof of concept
GxAlert
GxAlert is a software application designed to work with Cepheid's GeneXpert®. It enables real-time transmission of relevant data such as lab results, cartridge consumption, error rates and device malfunctions, from GeneXpert machines on the field to a real-time central online database that can be accessed anywhere. Reporting time is reduced from weeks to just seconds, facilitating effective oversight, disease surveillance, commodity management and performance management. It also allows for faster enrolment into care by automatically sending real-time notifications and alerts about diagnosis to the relevant program personnel.
The GxAlert was first designed and launched in 2012 in Nigeria. At the time, the country had recently introduced and was rapidly deploying GeneXpert machines to laboratories across Nigeria. The National Tuberculosis and Leprosy Control Programme (NTBLCP) was faced with the challenge of maximising the effectiveness and value for money of its GeneXpert investments. The exponential rise in DR-TB case detection ushered in by the introduction of the GeneXpert machine was becoming increasingly difficult to coordinate manually. Abt Associate, which was already supporting the NTBLCP on technology solutions, provided support to conceptualize a digital solution that would meet the country’s needs. This concept dubbed “GxAlert” was developed on behalf of Abt Associate by System One. Collaborating and obtaining buy-in from the responsible government entity was a critical first step in the success of GxAlert introduction in Nigeria. This was particularly relevant considering the myriad of implementing partners, donors and other players in the country’s GeneXpert implementation landscape. It was therefore prudent to key into the country’s GeneXpert Implementation Guideline, which aims to harmonize the GeneXpert implementation process across all stakeholders by providing a standardized framework coordinated by the NTBLCP.
Once the solution was developed, as a proof of concept, the GxAlert was piloted on 9 GeneXpert machines across three states for a period of three months. This was later expanded to 38 machines, by the end of 2014. The pilot proved to be successful; GxAlert improved notification rates, and end to end visibility of the laboratory network, enabling informed decision making at the central level and prompt treatment initiation. The success of the pilot was used by the NTBLCP and its partners to secure funding from USAID to scale up GxAlert to 200 GeneXpert machines. The pilot also formed the basis for upgrading the GxAlert software. Challenges, successes and lessons learnt were closely monitored throughout the pilot. The findings were then analysed and disseminated among stakeholders. Feedback was obtained after the pilot and used to update software to better meet the needs of the country. Multi-stakeholder participation and consensus on this process improved acceptability of the device, which facilitated uptake. Some of the improvements made include the capability of collecting and transmitting custom biodata such as patient age, sex, address and HIV status. An inventory module was also built into the GxAlert device to capture more appropriate information on cartridge utilization. At inception, the GxAlert was designed to transmit only MTB positive, Rif resistant results. It now has the capability of transmitting all types of results. Challenges with connectivity have also been addressed by replacing the single modem devices that were initially used to a dual sim router, such that a second mobile network kicks in, when the first one fails.
After the initial expansion support by USAID, the Global Fund also provided support for the installation of GxAlert on an additional 200 GeneXpert machines. This has ensured that GxAlert support has grown in tandem with GeneXpert installations in the country. Since 2015, a GxAlert device is always installed at the point of GeneXpert deployment to a laboratory. With the help of GxAlert, Nigeria’s DR-TB enrolment gap (i.e. gap between number of patients diagnosed and those enrolled on treatment) has increased from 53% in 2013 to 83% in 2018. The success of GxAlert in Nigeria has contributed to the increase in TB notification from 17% when it was first introduced to 25% today. The device has also found global application. GxAlert’s success in Nigeria has led to its roll out to 2,500 GeneXpert laboratories in 47 countries, delivering 7,000,000 TB results since 2012. It is also being used across different disease areas beyond TB.
Over the 7 years since it was launched, Nigeria has progressively benefitted more and more from the opportunities for GeneXpert optimization that GxAlert presents. It has however not been without its challenges. Due to interruptions in internet connectivity and power supply, the potential of GxAlert to replace manual, paper-based data transcription and reporting, cannot be fully exploited. For now, it is considered by the NTBLCP to be a secondary reservoir of data, rather than a primary reporting tool. In 2017, USAID transitioned its funding support for GxAlert in Nigeria to the Global Fund. Technical support is now being provided directly by the developers of the device, System One. The country is gearing up to upgrade to the newly improved version of the software called ASPECT. ASPECT will have improved connectivity across multiple laboratory equipment and with other data management tools such as the e-TB Manager.
WHO Relevant resource documents
WHO Global Observatory for e-Health – Nigeria Report
https://www.who.int/goe/data/country_report/nga.pdf
Nigeria Health Information System Policy
https://ehealth4everyone.com/wp-content/uploads/2015/09/Nig-Health-Info.pdf
National e-Health Strategy Toolkit
https://www.who.int/ehealth/publications/en/
A Literature Review of e-Health Sector and Challenges in Nigeria
References
https://cchubnigeria.com/aof/health/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3537795/
http://healthenabled.org/wordpress/nigeria-digital-health-dashboard/
https://www.researchgate.net/publication/272852712_Issues_on_E-health_Adoption_in_Nigeria
https://www.shopsplusproject.org/article/new-mobile-application-speeds-tb-referral-and-reporting