Philippines Country Profile
Philippines
105 Million
Purpose and intended audience
Landscaping of existing Digital TB Technology tools and new relevant products that may be applied across the TB Care Cascade Model, and providing insight to product developers and partners into country-specific policy, regulatory and implementation processes.
Key numbers
Country TB profile
TB Cases the Health System is Identifying:
57%
Health System Efficiency Ranking:
60
Business
Ease of Doing Business Ranking:
124
Economy
Income Category:
Upper LMI
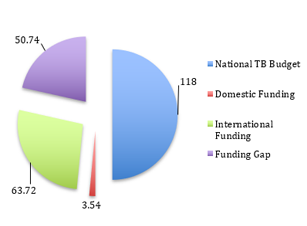
Budget & Financing
National digital TB landscape
Table 1: Digital TB Care Technology Tools currently in use
| PRODUCT NAME | DEVELOPER | USAGE |
|---|---|---|
| Diagnostocs & Transport | ||
| QUAN TB | MSH (SIAPS) | Electronic quantification and early warning system -supply planning for TB treatment. |
| QFT-Plus | QIAGEN | TB diagnostic software |
| Adherence | ||
| 99DOTS | Open Source | Low cost monitoring and improving medication adherence |
| Patient Support & Direct Benefits | ||
| MEDIX | MEDIX | Cloud-based clinic management software; medical records |
| OpenClinical | Open Source | Electronic health record system for local government health centers |
| SeriousMD | SeriousMD | Appointment Management, Electronic Medical Records, Billing, Analytics and more. |
| ICT, Dashboard & AI | ||
| PVIMS - [Pharmacovigilance Information Management System] | MSH (SIAPS) | Web-based application used by clinicians, regulatory bodies, and implementing partners to monitor the safety of medicines |
| ITIS [Integrated TB Information System] | web-based system and tool for data collection, processing reporting | |
| Vaccination | ||
| New Drug Regiment | ||
| Triage | ||
| Sequencing | ||
| Early Care Seeking | ||
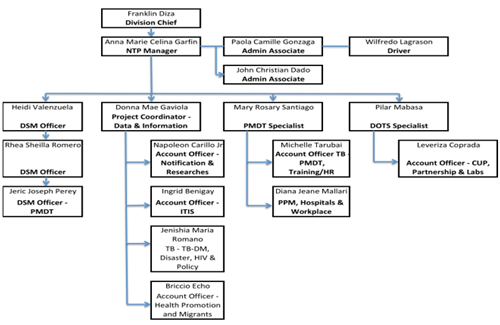
National TB country team
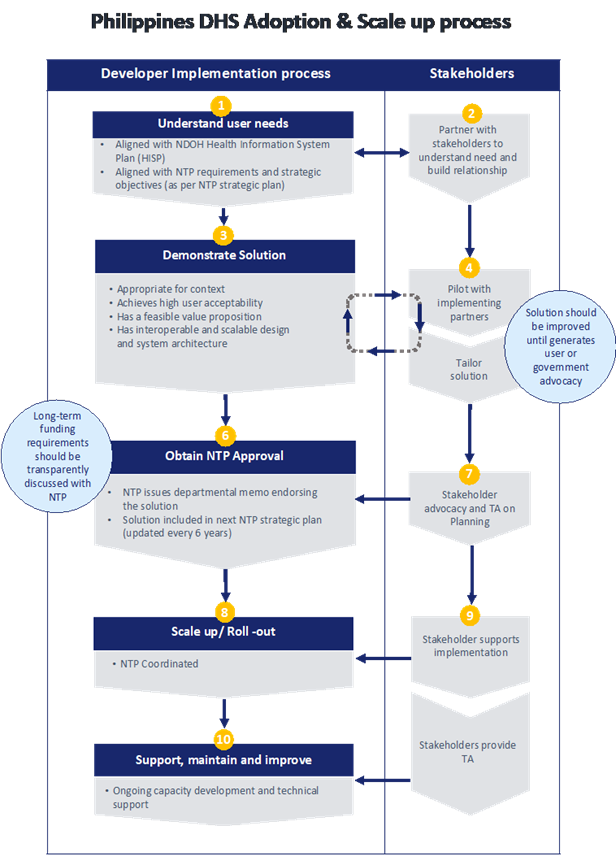
Market Entry Diagram
e-Health Policy & Regulation
THE PHILIPPINES NATIONAL e-HEALTH PROGRAM
(eHealth Legislation, Policy and Compliance)
Objectives:
- Formulate and adopt required national policies, legislations and protocols across health sector to support the attainment of the e-Health vision; review health sector segment policies for alignment and comprehensiveness; establish regular policy reviews and implementation monitoring. Examples of these are the national legislations, policies, and regulations on how health information are stored, accessed and shared across geographical and health sector boundaries; implementation of unique health identifier; implementation of national health data standards; and software certification or accreditation.
- Create and sustain a legal compliant and policy-driven environment to establish trust, buy-in and protection among consumers and industries involved in e-Health practices and systems.
Critical Targets:
- Push for the approval of an e-Health/Telemedicine/Telehealth Law.
- Institutionalization of the National e-Health Program.
- Formulation of the necessary policies, mechanisms and standard operating procedures for the use and adoption of health data standards and interoperability.
- Formulation of the necessary policies, mechanisms and standard operating procedures to establish/institutionalize health data privacy and security.
- Formulation of the necessary policies, mechanisms and standard operating procedures for the application/software compliance to DoH & PhilHealth reporting requirements, and related national standards.
- Formulation of the necessary policies, mechanisms and standard operating procedures to establish a secure health information exchange.
- Formulation of other related policies on administrative, legal, ethics, research, finance aspects of eHealth.
- Regular review and updating with stakeholders, considering international and national policies and commitments, various eHealth policies and protocols, plans, and frameworks.
Actual Accomplishments:
1.Draft e-Health Bill for the 17th Congress.
2.Draft Administrative Order on the Institutionalization of the Inter-Agency National eHealth Governance Committee of the National e-Health Program.
3.DOH Administrative Order No. 2013-0025: National Implementation of Health Data Standards for eHealth Standardization and Interoperability (eHSI Release 01)
4.DOH Administrative Order No. 2015-0037: National Implementation of Health Data Standards for eHealth Standardization and Information Interoperability
- Joint DOH-DOST-PhilHealth Administrative Order on the Implementation of the Philippine Health Information Exchange
- Joint DOH-DOST-PhilHealth Administrative Order on the Privacy Guidelines for the Implementation of the Philippine Health Information Exchange
7.J oint DOH-PhilHealth Administrative Order on the Adoption of the Philippine Health Information Exchange Lite
- Joint DOH-PhilHealth Administrative Order on the Implementation of the National eHealth Electronic Medical Record System Validation for the Various National Health Data Reporting Requirements – for formal endorsement of respective Legal/Policy Offices of the concerned agencies.
- DOH Administrative Order on the Institutionalization of the National Tele-Health Services under the Department of Health – ongoing revision of document.
10.DOH Administrative Order on the Implementation of the National Health Facility Registry – ongoing revision of document.
11.Memorandum of Agreement on the Management and Implementation of the Philippine Health Information Exchange (PHIE) and Other Related eHealth Projects signed last 17 March 2015.
12.Memorandum of Understanding on the Philippine National Public Key Infrastructure signed last 9 July 2015.
13.Resolution to Formally Manifest Before the Cabinet the Need to Establish the National Privacy Commission and Proposal for Creation of the National Health Data Privacy Board.National eHealth Information Interoperability Standards Change Management Manual – for final approval by the National eHealth Technical Working Group.
14.National eHealth Information Interoperability Standards Catalogue – for final approval by the National eHealth Technical Working Group.
Read more…
https://www.doh.gov.ph/Policies-and-Laws/ITIS-Implementing-Guidelines-rev
http://ehealth.doh.gov.ph/#close
MEDICAL DEVICE APPROVAL PROCESS
The Philippines medical device market is valued at US $300 million with a growth rate of 9% CAGR. Majority of the patients are form the private healthcare sector. The government has enforced the FDA Act of 2009 to strengthen the regulations of medical products in the Philippines.
There are two key documents that are required for registration:
- License To Operate (LTO)
- Product Registration
- License to Operate (LTO)
Any establishment in the Philippines from single proprietorship to public and private limited companies can apply for the LTO as long as they are engaged in the healthcare field as importers/ distributors/wholesalers/manufacturers/traders etc.
The documents required to get an LTO for a medical device importer / distributor are:
- Business Registration Certificate
- Location Plan
- Contract of lease of Space
- Pharmacist Information
- Product Listing
- Floor Plan
- Agreement with Medical Device Manufacturer for Distribution
Time frame to obtain the LTO is between 1 – 3 months.
- Product Registration
A company that has a LTO can apply for the registration certificate. Philippines FDA classifies instruments, apparatus, machines, appliances, implants, in-vitro diagnostics, software and any material or article which is used in the diagnosis, prevention, monitoring, treatment or alleviation of diseases and does not achieve its primary function in or on the human body by pharmacological, immunological or metabolic means.
The classification of medical devices in the Philippines is of two types:
- Registerable
- Non- Registerable – A Certificate of Exemption will be issued by the CDRRHR for non-registerable devices after the evaluation of your documents.
Key Documents required getting the medical device registration certificate in the Philippines:
- ISO 13485
- Free Sale Certificate from Country of Origin
- Device description, features, indications
- Instructions for Use
- Pre-clinical Studies
- Clinical Studies
- Shelf Life and Biocompatibility
- Label
- Risk Analysis
- Manufacturing Process
Time taken to obtain approval on submission of dossier is 6-9 months with a validity of 1 or 5 years.
PUBLIC PROCUREMENT PROCESS & TENDERS
The Philippine Government Electronic Procurement System or PHILGEPS is the single, centralized electronic portal that serves as the primary and definitive source of information on government procurement. The PHILGEPS website provides guidance on participating in a government bid. (https://www.philgeps.gov.ph/)
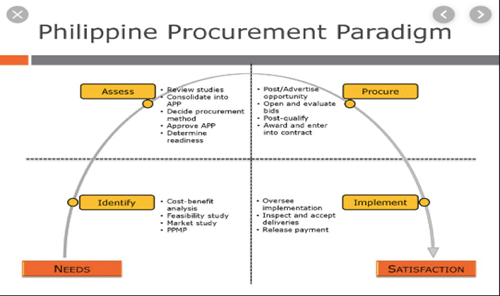
Procurement System In Health In The Philippines:
https://www.slideshare.net/TedHerbosa/procurement-system-in-health-in-the-philippines
Manual for the Procedures for Procurement of Goods & Services in The Philippines:
https://www.gppb.gov.ph/downloadables/forms/GPM%20-%20Vol.2.pdf
Operational issues & challenges
The Philippine medical equipment market remains a lucrative one for suppliers. Market demand is driven by the private sector, where three major hospital developers – Metro Pacific Investments Corporation (MPIC), QualiMed Health Network (Qualimed), and Mt. Grace Hospitals, Inc. (MGHI) – continue to expand through acquisitions of existing facilities (MPIC and MGHI) and through construction/development of new hospital projects (Qualimed). Major factors impacting demand are population growth, steady economic growth at 6.7% (2017 est.), and hospital expansion and upgrading.
The market is price-sensitive, which explains the growing presence of inexpensive medical equipment from China or South Korea.
The Philippines participates in the ASEAN Medical Device Regulatory Harmonization efforts, an initiative that aims to improve and standardize the medical device regulatory process in the region. Its ultimate objective is to facilitate the regulatory process for medical device registration in ASEAN member countries.
Suppliers interested in selling medical equipment/devices (incl. SaMD) in the Philippines should appoint a local distributor who will handle all aspects of importation including registration, obtaining a license, and getting customs clearance for the products. The local distributor not only helps facilitate the product's entry into the market, but also assumes responsibility for advertising and promotion through sales and dealer networks. He/she registers with the Food & Drug Authority (FDA) before operating and receives a License to Import and a License to Operate (LTO) from this agency FDA.
Current demand reflects healthcare requirements for growing incidences of hypertension, diabetes/kidney diseases, TB/respiratory ailments, and cancer. Products with high sales potential for U.S. suppliers include electro-cardiographs, computed tomography apparatus (CT scan), magnetic resonance imaging (MRI) equipment, ultrasonic scanning machines (ultrasound), X-ray and radiation equipment, breathing appliances, and linear accelerators. Demand for diagnostic laboratory products, supplies, and biological rapid test kits also exist.
The Government has not released a final list of products classified as medical devices and it will fall on each firm to research and determine whether their product will fall into the medical device category in the Philippines. Firms that are unsure can submit an application to have their product classified.
Since March 2019, medical device imports (incl. SaMDs) are subject to existing FDA regulations and request the FDA’s Center for Device Regulation, Radiation Health and Research (CDRRHR) to issue a Certificate of Exemption (COE) to facilitate the release of imported medical equipment from Bureau of Customs custody.
Read more…https://2016.export.gov/industry/health/healthcareresourceguide/eg_main_108611.asp
Medical Device (including SaMD) Registration service providers
https://www.asiaactual.com/philippines/medical-device-registration/
https://www.andamanmed.com/philippines/
https://www.arqon.com/product-registration-asean-philippines
Healthcare resource guide for Philippines [market entry, market trends, current demand; registration process…]: https://2016.export.gov/industry/health/healthcareresourceguide/eg_main_108611.asp
Case studies / Proof of concept
Introduction and rollout of PViMS in Phillipines
Through a partnership with Janssen Therapeutics of Johnson & Johnson that began in 2015, USAID introduced a program to distribute a new medicine called bedaquiline that helps patients with MDR-TB in low-income countries including the Philippines. The USAID-funded Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Program, which Management Sciences for Health (MSH) leads, has been helping to roll out bedaquiline in low- and middle-income countries. But with any new treatment, active pharmacovigilance (PV) is needed to help ensure both patient safety and drug effectiveness. This means being able to monitor patients to identify and evaluate adverse events, such as unexpected or serious side effects, to better understand possible risks and improve treatment protocols. However, when SIAPS assessed the Philippines’ PV monitoring system, it found a number of weaknesses, particularly for programs that address MDR-TB. The monitoring system lacked PV-specific data collection and analysis tools and up-to-date safety alerts from regulatory agencies. The patient management tool utilized by the Philippine government was out-of-date and lacked needed functionality.
To address these gaps, the SIAPS team in the Philippines partnered with the Department of Health’s (DOH) Pharmaceutical Division, Food and Drug Administration, and the National TB Control Program to strengthen drug safety monitoring and management activities. This included helping the agencies implement the Pharmacovigilance Monitoring System (PViMS), a web-based application SIAPS developed to help clinicians, regulatory bodies, and implementing partners monitor medicine safety specifically in resource-limited countries.
SIAPS supported development of guidelines for using the app in the country and held its first PV workshop last month. The participants—63 people from 9 regions—represented central and regional offices from the various government partners. Representatives also included staff from the 10 health care facilities that piloted a new shorter treatment regimen for MDR-TB.
The group discussed ways to strengthen PV coordination, recording, reporting, and health worker capacity-building in their respective reporting systems. Each region drafted plans to implement one of the key activities of active TB drug-safety monitoring and management (aDSM) reporting per WHO guidelines or introducing bedaquiline treatment.
In order to jumpstart the adoption and rollout of PViMS and help integrate pharmacovigilance in public health programs nationwide,” PViMS introductory workshop was organized by SIAPS and Philippines Government relevant bodies (see above). The workshop promoted PViMS as a valuable tool to the existing health system in the Philippines, which helps to ensure that available TB medicines in country are safe and effective, but also, eventually, would help the Philippine government attain the best possible health outcomes for its people.
PViMS has also been slated for use by other DOH programs in the Philippines, such as malaria, HIV/AIDS, immunization, family planning, maternal and child health, and nutrition, to ensure patient safety for medicines being used in these programs.
The app can be accessed on the Philippine DOH’s website.
The pilot started in 2015-2016 with initially 10 health facilities piloting PViMS; by the end of 2018, PViMS had been expanded to 9 regional facilities in the Philippines.
WHO Relevant resource documents
WHO Global Diffusion on e-Health – Report [https://apps.who.int/iris/bitstream/handle/10665/252529/9789241511780-eng.pdf?sequence=1]
National e-Health Strategy Toolkit
https://www.who.int/ehealth/publications/en/
Regional Strategy for Strengthening e-Health in South-East Asia Region – WHO (2014 – 2020)
http://www.searo.who.int/entity/health_situation_trends/ehealth_strategy.pdf?ua=1
References
http://ehealth.doh.gov.ph/#close
https://www.manilatimes.net/government-launches-ehealth-system/73931/
https://www.slideshare.net/ExistPH/e-health-for-health-it-conference
https://www.slideshare.net/amarcelo/country-ehealth-updates-2016-philippines
https://www.slideshare.net/isiptan/healthcare-technology-in-the-philippines
https://opinion.inquirer.net/112263/wanted-leaders-tb-free-ph
https://supportoffice.jp/outreach/2013/philippines/1-3_doh_phil.pdf
https://morulaa.com/medical-device-product-registration-philippines/
https://www.msh.org/resources/quantb
http://siapsprogram.org/tools-and-guidance/pvims/
http://www.openclinical.org/os_chits.html
https://www.stlukes.com.ph/procedures/quantiferon-tb-gold-test
https://www.doh.gov.ph/Policies-and-Laws/ITIS-Implementing-Guidelines-rev