Vietnam Country Profile
Vietnam
96 Million

Purpose and intended audience
Landscaping of existing Digital TB Technology tools and new relevant products that may be applied across the TB Care Cascade Model and providing insight to product developers and partners into country-specific policy, regulatory and implementation processes.
Key numbers
Country TB profile
TB Cases the Health System is Identifying:
85%
Health System Efficiency Ranking:
160 of 187
Business
Ease of Doing Business Ranking:
69 of 190
Economy
Income Category:
Lower Low Middle Income
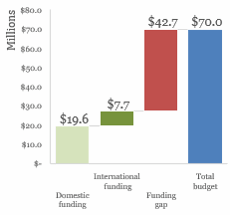
Budget & Financing
National digital TB landscape
Table 1: Digital TB Care Technology Tools currently in use
| PRODUCT NAME | DEVELOPER | USAGE |
|---|---|---|
| Diagnostocs & Transport | ||
| Grab App | Grab Holdings | Transporting TB suspects for screening |
| QUAN TB | MSH | Quantification & Supply Planning |
| Early Care Seeking | ||
| GxAlert | Open Source | Connectivity |
| ICT, Dashboard and AI | ||
| DHIS-2 - software | Open Source | Reporting, analysis and dissemination of health program data. |
| Adherence | ||
| ACIS | Open Source | Improving Patient Linkage to care |
| Triage | ||
| VITIMES | NTP Vietnam | Information Management |
| e-TB Manager | MSH | Case Management |
| eCDS | Viettel | Reporting System |
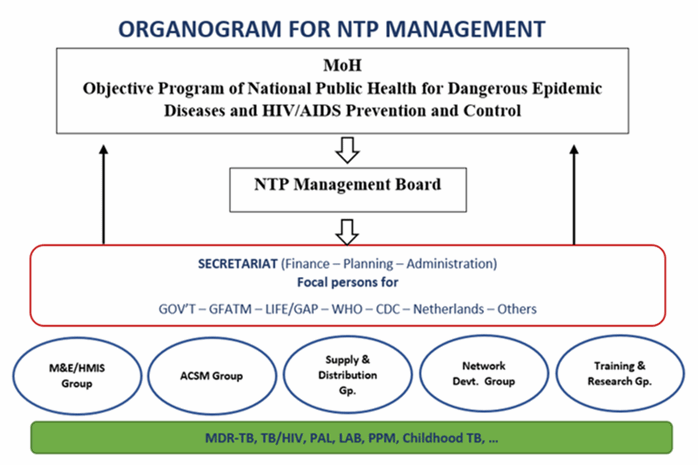
National TB country team
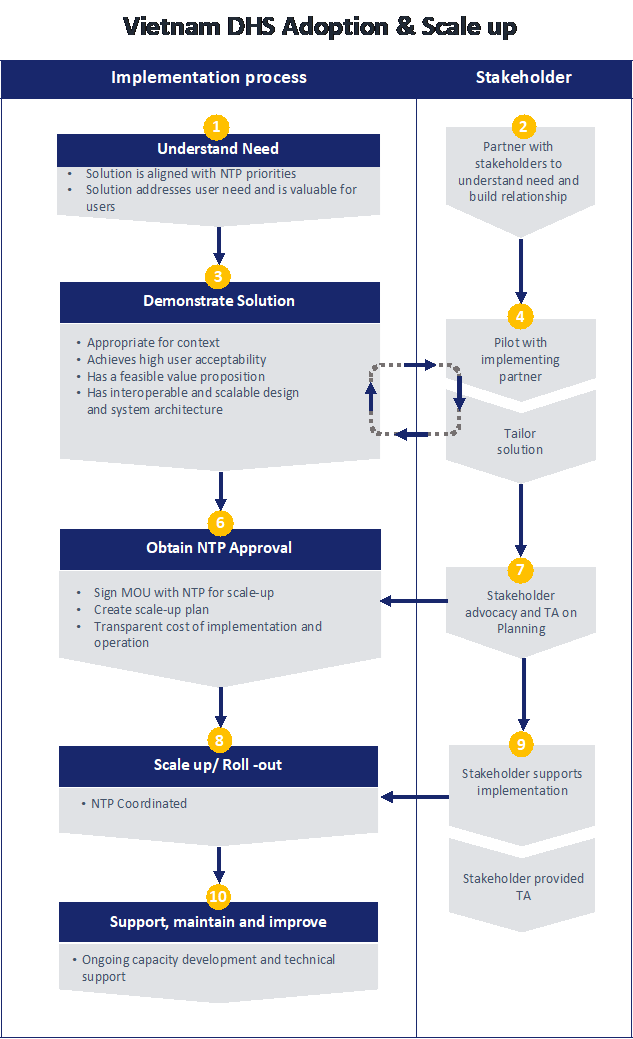
Market Entry Diagram
e-Health Policy & Regulation
eHealth implementation in Vietnam is guided by the National Health Information Strategic Plan (2011-2015). No updates have been made recently
To be marketed in Vietnam, medical devices must be registered with the Ministry of Health (MoH) or the appropriate provincial department of health. Each different product must be separately registered, at a cost of $2,000/product. The approval process usually takes from six months to one year but can take longer. To register a product requires a Certificate for Sale (CFS), Good Manufacturing Practices (GMP) approval from the Institute of Quality Control, and sample testing from five different committees. All documentation must be complete and accurate. Medical documents and related material must be in both English (or French) and Vietnamese, and notarized. These materials should be prepared by a qualified Vietnamese individual, preferably a doctor. If approved, the resulting license is valid for two years. Most private companies and local state-owned companies will be familiar with registration requirements, procedures and paperwork, and can handle applications easily. It is strongly recommended that foreign companies seeking to import and market medical devices in Vietnam arrange to have a local partner with strong technical skills and good connections with the MoH, hospitals and other healthcare facilities.
To import medical devices into Vietnam, an importer must have a Bill of Lading, Certificate of Authority, and specific individual licenses, all from the government. The government is reported to have become strict about documentation, and approvals are delayed significantly if there are paperwork errors. All imported medical equipment must go through a government owned trading company that has a license for direct importation. These import companies can also act as distributors and wholesalers. There are 10 such licensed companies, including YTECO, SAPHACO, VIMEC 2 in the south of the country, and VIMEC 1, VIMEDIMEX 1 in the north. These companies normally charge the equipment purchasers or distributors 1% – 2% of the total value of the imported goods as a commission for services. In general, importation procedures take about 2 – 3 weeks, and there often major difficulties that delay the process. If a foreign company does not have a representative office in Vietnam, such a company’s local distributor/agent is responsible for registering the imported medical equipment with the MoH. If there is a representative office, that office is responsible for registration.
Medical devices in Vietnam are classified and managed based on their potential risks, as determined by qualified entities. It is important that suppliers understand the regulatory requirements for the classification risk of the software being registered. On December 31, 2018, the Vietnamese government promulgated Decree No. 169/2018/ND-CP (Decree 169), amending Decree No. 36/2017/ND-CP (Decree 36) on medical device management. In the latest decree 169/2018/ND-CP, which came into effect in January 2019, software for medical purposes is now exempt from registration and import licensing. However, foreign classification results are no longer accepted in Vietnam. Instead, the results must come from government-qualified local agents, some of whom still do not have appropriate capacity to conduct medical device classifications. Inaccuracies in classification results have been known to occur.
Registration procedures for imported medical equipment are as follows:
- Submission of a Permit Certificate application to sell medical equipment, including:
a. The application form itself;
b. Legal documents from the country of origin certifying that that foreign country has permission to sell the medical equipment in question; and
c. Documents certifying the foreign company is a Vietnamese legal entity; and - Submission of an application for a License for Circulation of Medical Equipment in Vietnam, including:
a. The application form;
b. A list of the medical equipment to be circulated;
c. A certificate of product quality, issued by the country of origin;
d. A catalogue with detailed use instructions for each product; and
e. The results of clinical tests for each product.
PUBLIC PROCUREMENT PROCESS & TENDERS:
Key legal documents forming the legal framework for public procurement bidding activities in Vietnam include:
- Law No. 61/2005/QH11 on Bidding, adopted by the National Assembly on 29 November 2005, which took effect 1 April 2006
- Decree No. 85/2009/ND-CP providing guidelines for the implementation of the Bidding Law and for the selection of construction contractors pursuant to the Law on Construction issued by the Government on 15 October 2009 (“Decree No. 85”); and
- Circular No. 17/2010/TT-BKH providing details for pilot online bidding issued by the Ministry of Planning and Investment on 22 July 2010 (“Circular No. 17”).
Although Vietnam has become a member of several international organizations, including the World Trade Organization (“WTO”) and the Association of Southeast Asian Nations (“ASEAN”), Vietnam has not made any commitments to any agreement on government procurement under such organization (for example, the WTO Government Procurement Agreement (“WTO GPA”)). However, the WTO GPA is being studied by the Ministry of Planning and Investment and the National Committee for International Economic Cooperation in Hanoi in order to introduce WTO GPA to Vietnamese enterprises over time and to make initial preparations for eventual WTO GPA accession.
Basic principles underlying Vietnam’s bidding legal framework are fairness and transparency. These principles, which can be found in provisions on bidding information (which requires certain kinds of information to be published in a bidding newsletter and on the bidding website of the State bidding administrative body), include: assurance of competitiveness in bidding process; prohibited acts in bidding; provision on currency to be used in bidding (in Vietnam, the currency to be used in bidding should be stipulated in the bidding invitation documents on the principle of one currency for one specific volume); and all domestic costs must be quoted in Vietnamese dong.
The procurement procedures include the following:
- Bidding plan
- Bid implementation documentation
- Preparation of bids
- Bid opening
- Bid evaluation
- Bid results
Details of Vietnam’s procurement process and tender can be accessed at:
https://vietnamtendering.wordpress.com/2013/04/12/qa-vietnam-public-procurement-legislations/
Operational issues & challenges
Vietnam is well positioned to adopt digital health solutions. With steady economic growth, a growing population and a rapidly expanding middle class, the government of Vietnam is driving a digitalisation agenda in hospitals and clinics across the country. Smart solutions are being strongly encouraged to help alleviate Vietnam’s healthcare challenges and increase quality of care.
However, there are several challenges for new market entrants. For instance, procurement processes in public hospitals can be complicated and time-consuming. Therefore, partnering with local IT service providers or medical device distributors can provide faster access to the Vietnamese market including its decision makers, distribution networks and end-user contacts. Local partners can also help to overcome language barriers, share working capital and draw on their expertise in local licensing procedures.
To succeed in the Vietnamese market, it is important to consider several important factors such as: adapting to the local business culture, finding the right local partners and staff and ensuring the adaptability of products to the local market. Companies should invest resources to raise awareness of digital solutions. This, in turn, will drive a change in consumer perception and behaviour. Companies should also invest time in understanding the healthcare sector in Vietnam, including its people, processes and technologies. Cultural considerations are also important. Business relationships are formal in nature and can take time to develop. Meanwhile, meetings are an integral part of the business process. People often prefer to meet their prospective partners face-to-face. Other challenges in the market include bureaucratic procedures, unclear legislation and a lack of intellectual property rights enforcement.
To improve the quality of healthcare service, the government encourages both public and private investments in developing or expanding healthcare facilities, training medical professionals, implementing IT solutions, and the management of healthcare facilities.
Vietnam is not a market for inexperienced exporters. Foreign companies preparing to enter the Vietnamese market must plan strategically and be persistent and consistent with face-to-face follow-up. It can take one or two years to make a successful sale in this market. Building relationships is critical to success. Newcomers entering the Vietnamese market will need to consider two marketing strategies; one for the northern part of the country, which has a higher concentration of government ministries and regulatory agencies, and one for the south, which is the dominant industry hub. The two markets also differ in terms of consumer behaviour and preferences. To enter or expand in Vietnam, foreign companies may do so indirectly through the appointment of an agent or distributor. It would be prudent to conduct sufficient due diligence on potential local agents/distributors to ensure they possess the requisite permits, facilities, manpower, and capital. Firms seeking a direct presence in Vietnam should establish a commercial operation utilizing the following options: a representative office license, a branch license, or a foreign investment project license under Vietnam’s revised Foreign Investment Law.
Case studies / Proof of concept
Improving Patient Linkages in Vietnam to Reduce Loss to Follow-Up
In most Vietnamese health facilities, patient referrals are time-consuming and poorly managed, qualities that significantly contribute to gaps in treatment enrolment. Health workers handwrite referral instructions and rarely follow up with their patients to check if they actually arrived at the referred facilities. There is no formal or routine reporting system to monitor the number of patients that have arrived, except by manually comparing logbooks or by comparing aggregated counts.
To meet this gaping need, the Clinton Health Access Initiative (CHAI) led the design and development of an SMS-delivered web-based referral and treatment support system in 2014 called Access to Care Information System (ACIS) to improve communications between facilities and patients. It was designed to be simple and easy to use, requiring minimum patient data and minimum burden to the health worker. The software was coded using the most common open source platform (PHP/MySQL), that has a plethora of programmers who understand the language. The software is hosted on government servers, promoting sustainability and data ownership. CHAI’s relationship with the Government of Vietnam and other key stakeholders garnered from its trusted position as technical partner, and CHAI’s familiarity with the country landscape played a significant role in facilitating introduction and acceptance of ACIS.
ACIS implementation commenced in 2014 with a pilot in Yen Bai province two HIV testing sites and two OPCs, resulting in a 50% reduction in loss to follow up. Average time to successful referral was reduced from 17 days to 10.5 days. This success led to an expansion of ACIS to 112 testing and treatment facilities in 8 provinces. By the end of 2014, ACIS had delivered impressive results by halving lost to follow-up from 43% to 21%. The success of the pilot sparked strong interest for the tool, as well as the support of the Ministry of Health (MoH) and partners. By May 2015, ACIS had expanded to over 300 HIV and TB testing and treatment facilities in 25 provinces. The ACIS system has continued to improve based on feedback from end users and key stakeholders. Over the last 3 years, ACIS has been implementing the following changes:
- Scaling up ACIS to all 1,411 TB and HIV testing and treatment facilities
- Developed a mobile phone version of the tool
- Expand coverage to other disease areas and
- Improving interoperability with other digital platforms.
Furthermore, the software has now been linked to the VITIMES system. VITIMES data on cases of TB detected is used via the ACIS to identify, communicate with and screen contacts including household members and neighbours for TB.
WHO Relevant resource documents
WHO Global Observatory for e-Health – Vietnam Report
https://www.who.int/goe/data/country_report/vnm.pdf?ua=1
National Health Information Strategic Plan (2011-2015
https://thuvienphapluat.vn/archive/Quyet-dinh-2360-QD-BYT-nam-2011-phe-duyet-Ke-hoach-tong-the-ung-dung-phat-trien-vb150519.aspx
National e-Health Strategy Toolkit
https://www.who.int/ehealth/publications/en/
A Literature Review of eHealth Sector and Challenges in Nigeria
https://www.researchgate.net/publication/310809745_A_literature_review_of_eHealth_sector_and_challenges_in_Nigeria
World Health Organization (2017) Handbook for the use of digital technologies to support tuberculosis medication adherence
https://apps.who.int/iris/bitstream/handle/10665/259832/9789241513456-eng.pdf
World Health Organization (2018) WHO compendium of innovative health technologies for low-resource settings, 2016-2017.
References
- United States of America Department of Commerce (2019) Healthcare Technologies Resource Guide: A Reference for U.S. Exporters (2019) Vietnam Pages 246 – 248
https://build.export.gov/build/idcplg?IdcService=DOWNLOAD_PUBLIC_FILE&RevisionSelectionMethod=Latest&dDocName=eg_main_124814 - Australian Trade and Investment Commission (2019) Digital Health in Vietnam. A Guide to Market
https://www.austrade.gov.au/ArticleDocuments/4569/Digital%20Health%20in%20Vietnam%20Report.pdf.aspx - Hoang, T.T.T., Nguyen, N.V., Dinh, S.N. et al. BMC Public Health (2015) 15: 980.
https://doi.org/10.1186/s12889-015-2338-5
- PATH (2017) Using mHealth to combat multidrug-resistant tuberculosis across Vietnam
https://path.azureedge.net/media/documents/ID_vietnam_mobile_tb2017_fs.pdf
- Clinton Health Access Initiative (2015) Case Study: Improving Patient linkages in Vietnam to reduce loss to follow up