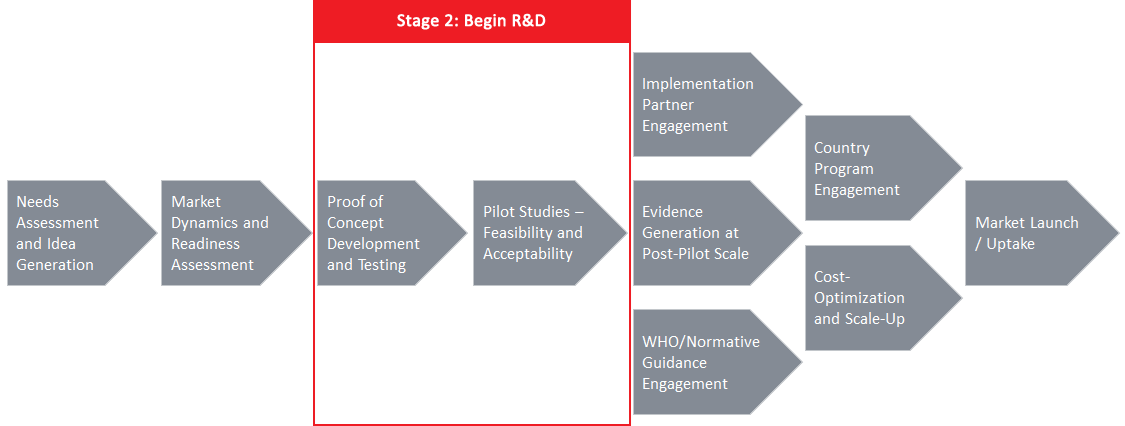
Stage Elements
- Develop user segmentation
- Update and strengthen patient/provider needs through market research or human-centered design
- Develop, test, and refine prototypes
- Conduct feasibility and acceptability evaluations
- Conduct effectiveness evaluations
- Evaluate development costs [and cost effectiveness]
- Develop business plan, including demand forecast
- Develop production and distribution strategies
- Develop plan for communications, training, advocacy, and key opinion leader engagement
- Assess country-specific regulatory landscape
- Identify and map stakeholders
Questions to Answer
- What do we hope to accomplish?
- Who needs to be involved?
- How will we manage the design process?
- How will we produce prototypes and get them into the field for early evaluation?
- Does our innovation “work” in real-world settings?
- Is our innovation well accepted by patients and providers?
- What criteria or features must/should our innovation include?
- How will we develop and distribute our innovation – at small, medium, and large scale?